
Andrew Trunsky on May 25, 2021
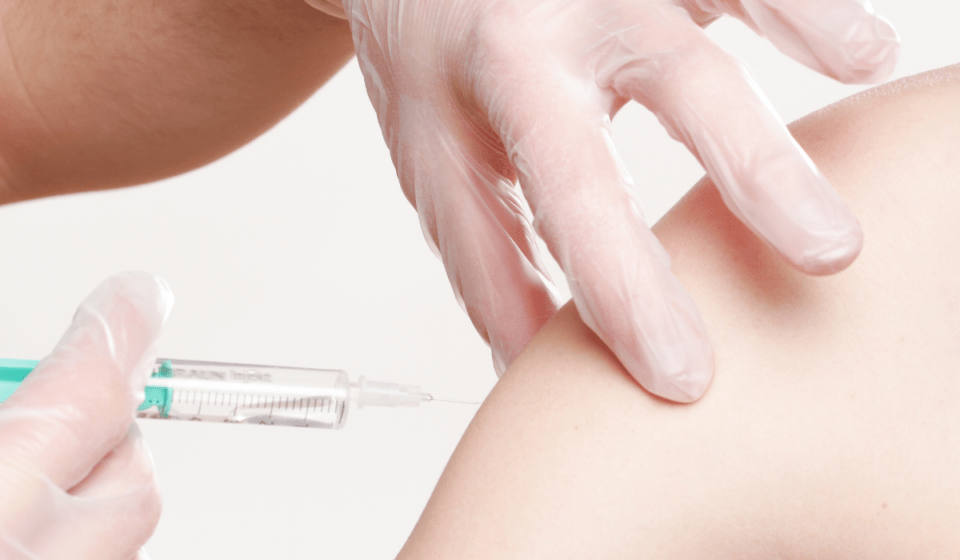
Moderna announced Tuesday that its coronavirus vaccine was 100% effective against infection in kids aged 12 to 17, and that it planned to apply for FDA authorization in June.
The company tested its vaccine in 3,732 participants in the age range, and found no cases of COVID-19 among those who received two doses or any “significant safety concerns” throughout its study. It was 93% effective two weeks after the first dose, and fully effective two weeks after the second, Moderna said.
“We are encouraged that mRNA-1273 was highly effective at preventing COVID-19 in adolescents,” Moderna CEO Stéphane Bancel said in the announcement. “We will submit these results to the U.S. FDA and regulators globally in early June and request authorization. We remain committed to doing our part to help end the COVID-19 pandemic.”
Moderna’s vaccine was first granted emergency use authorization for adults in December, one week after Pfizer’s became the first to be authorized in the United States. Trials for both two-dose vaccines have shown them to be overwhelmingly effective, with rates around 95%.
Pfizer’s vaccine, which was originally approved for people 16 and older, was approved for adolescents ages 12 to 15 two weeks ago.
Content created by The Daily Caller News Foundation is available without charge to any eligible news publisher that can provide a large audience. For licensing opportunities of our original content, please contact licensing@dailycallernewsfoundation.org.




2 comments
… [Trackback]
[…] Find More on that Topic: thelibertarianrepublic.com/moderna-says-vaccine-is-100-effective-in-teens-plans-to-apply-for-fda-authorization/ […]
… [Trackback]
[…] Find More to that Topic: thelibertarianrepublic.com/moderna-says-vaccine-is-100-effective-in-teens-plans-to-apply-for-fda-authorization/ […]